What is paralytic ileus in Dogs?
- Dr Andrew Matole, BVetMed, MSc

- Sep 28, 2025
- 18 min read
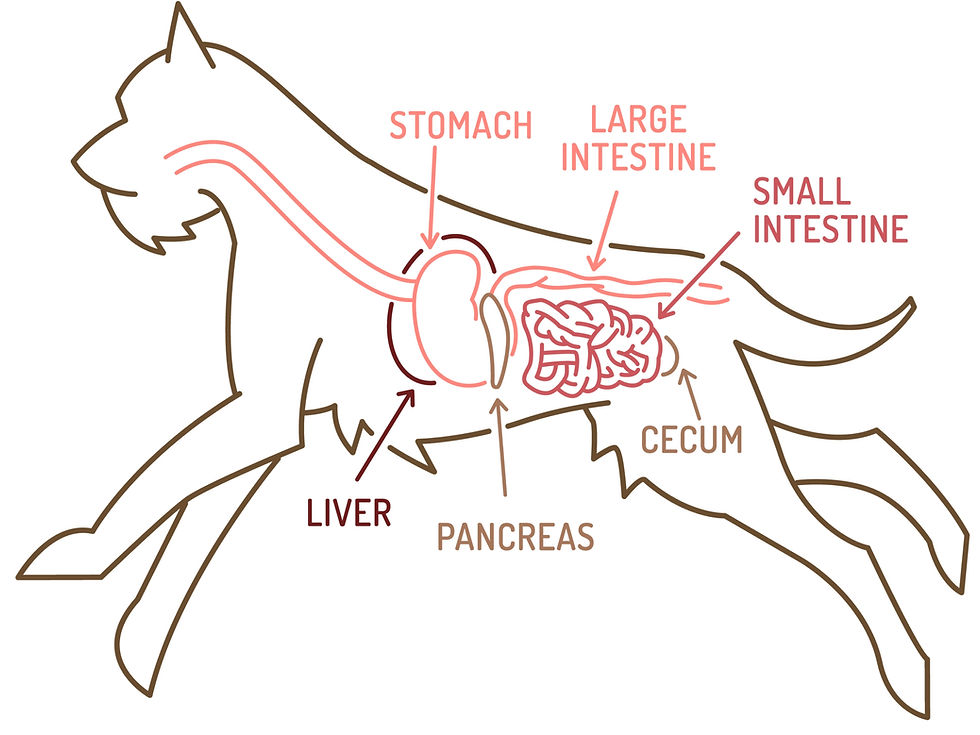
Paralytic ileus, also referred to as functional or adynamic ileus, is a clinical gastrointestinal (GI) disorder characterised by a temporary, reversible, or persistent impairment of intestinal motility resulting in a functional obstruction in the absence of a primary mechanical obstruction. In companion animals, such as dogs and cats, the normal, wave-like contractions of the intestine, known as peristalsis, are either absent or significantly reduced, leading to the accumulation of gas and fluid within the gastrointestinal (GI) tract, which causes bloating, pain, and other gastrointestinal signs. This stasis mimics the symptoms of a physical blockage, but the core issue is a functional paralysis of the intestinal musculature and its corresponding nervous control. It is crucial to recognise that ileus is not a primary disease itself but rather a secondary complication of abdominal inflammation or a clinical manifestation of an underlying systemic disease, condition, or trauma affecting intestinal motility. This distinction is fundamental to diagnosis and subsequent therapeutic strategy. The condition is commonly encountered postoperatively, and early recognition and intervention are vital in preventing severe morbidity or mortality (Hall & German, 2022).
What is Different between Paralytic Ileus and Mechanical Ileus?
The initial clinical challenge in patients presenting with signs of intestinal obstruction is distinguishing between paralytic ileus and mechanical ileus. Mechanical ileus results from a physical obstruction within the intestinal lumen, such as an ingested foreign body, neoplasia, or post-surgical adhesions (Mallo et al., 2021). This condition is considered a surgical emergency because of the risk of bowel ischemia, perforation, and necrosis (Ten Broek et al., 2018). In contrast, paralytic ileus is characterized by a functional impairment of peristalsis without the presence of a physical barrier (Vather et al., 2015).
Clinically, patients with mechanical obstruction often exhibit colicky abdominal pain and hyperactive bowel sounds (borborygmi) during the early stages as the intestine attempts to overcome the blockage, followed by diminished motility as the disease progresses (Di Saverio et al., 2016). Conversely, paralytic ileus typically presents with a quiet, aperistaltic abdomen and minimal or absent colicky pain (Vather & Bissett, 2013). This clinical distinction is critical, as mechanical obstruction generally necessitates prompt surgical intervention, whereas paralytic ileus is primarily managed conservatively through medical therapy and supportive care (Chapman et al., 2020).
What are the Mechanisms of Paralytic Ileus?
Neuromuscular Basis of Peristalsis

Normal gastrointestinal motility (GI) is an intricate and highly coordinated process governed by the enteric nervous system (ENS), often referred to as the "second brain" of the body. This system includes the myenteric (Auerbach's) and submucous (Meissner's) plexuses, which act as local control centers for intestinal function, coordinating smooth muscle contractions and secretion (Furness, 2012).

The coordinated propulsion of ingesta is achieved through a delicate balance between excitatory neurotransmitters, such as acetylcholine (acting on M3 receptors) and serotonin (via 5-HT4 receptors), and inhibitory mediators including nitric oxide and norepinephrine (Grundy et al., 2018). The result is a propulsive, aboral movement of contents, a process known as peristalsis. Paralytic ileus represents a profound disruption of this physiological equilibrium, resulting in a functional motor paralysis where the intestine fails to transmit these essential peristaltic waves, even when the underlying muscle and nerve cells are not structurally damaged (Vather & Bissett, 2013).
Biphasic Pathogenesis of Postoperative Ileus (POI)

Postoperative ileus (POI), one of the most common forms of paralytic ileus, is a multifactorial condition with a well-described, two-phase pathophysiological cascade. The initial phase is neurogenic, commencing with the surgical incision and tissue manipulation. This trauma triggers a neurogenic response, sensed by vagal afferent nerves, which transmit signals to the central nervous system, leading to a shift toward sympathetic nervous system control. This increase in adrenergic stimulation results in a rapid and temporary inhibition of intestinal motility (Wehner et al., 2012).
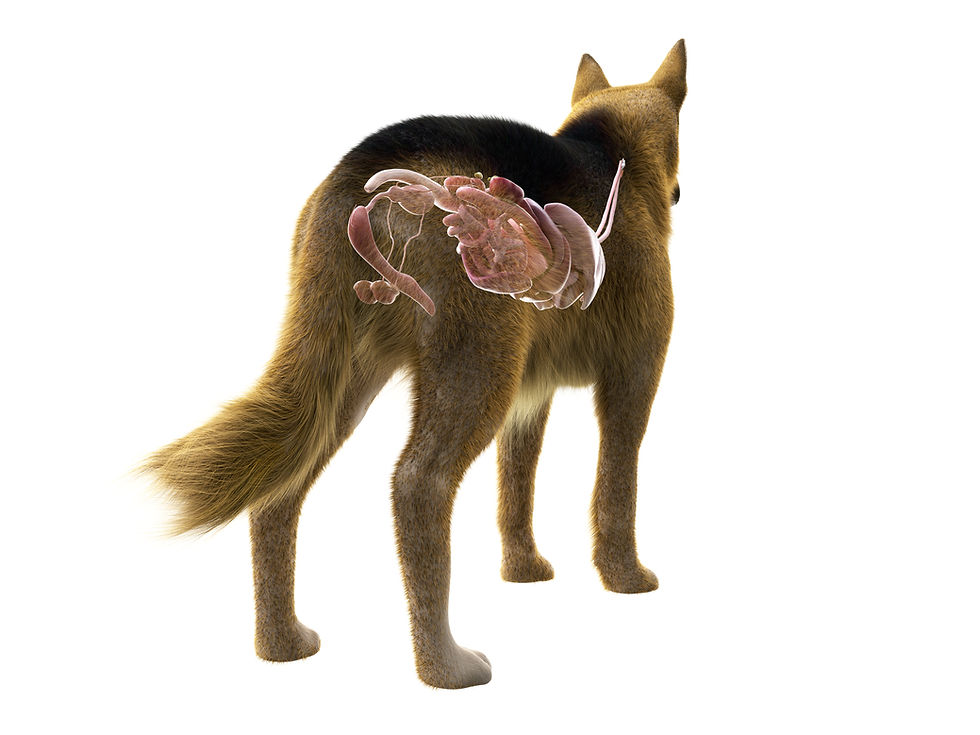
This neurogenic phase is then propagated and sustained by an inflammatory phase. Tissue manipulation activates enteric glial cells, which are a critical component of the enteric nervous system. These glial cells produce inflammatory products, such as nitric oxide and cytokines, which impair GI

contractility and lead to the formation of oedema within the intestinal wall, causing impaired contractility (Sharkey, 2015). This inflammatory process is self-perpetuating, as the resulting breakdown of the intestinal mucosal barrier allows for the translocation of luminal bacteria and their toxins into the systemic circulation. This phenomenon, known as bacterial translocation, exacerbates the systemic inflammatory response, perpetuating ileus (Kalff et al., 2013).
Etiological Spectrum and Systemic Interplay

While POI is a well-studied model, paralytic ileus can arise from a broad spectrum of other conditions. Systemic and metabolic derangements are a frequent cause. Electrolyte imbalances, in particular, play a significant role. Imbalances such as hypokalaemia, hyponatraemia, and hypochloraemia significantly impair neuromuscular transmission required for peristalsis (Kadam et al., 2019). Other systemic insults, such as septicemia from gram-negative bacteria, shock, blunt abdominal trauma, and organ failure (e.g., renal failure), are also known to precipitate ileus (Miedema, 2016).
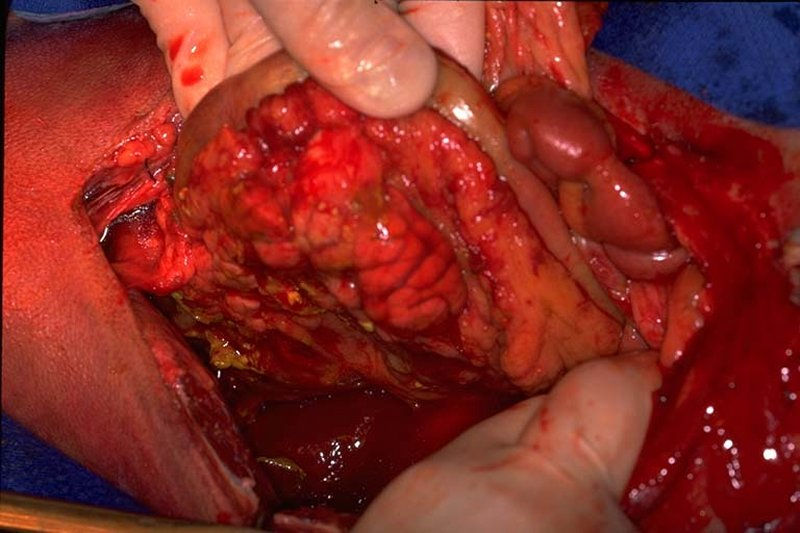
Furthermore, inflammatory and infectious diseases, whether localized to the abdomen or systemic, can directly lead to GI hypomotility via cytokine cascades. Conditions like enteritis, pancreatitis, and peritonitis trigger an inflammatory cascade that impairs normal intestinal function. Certain pharmacological agents and toxins also contribute; the use of opioids, for example, is a well-documented cause of ileus due to their direct inhibitory effects on GI motility (Luckey et al., 2003).

The relationship between intestinal function and systemic health is not unidirectional. A simple progression can demonstrate this complex interplay: a primary systemic insult, such as hypokalemia or septicemia, can directly inhibit the neuromuscular control of GI motility, causing a functional ileus. This results in gastrointestinal stasis and fluid accumulation then creates a permissive environment for bacterial overgrowth.
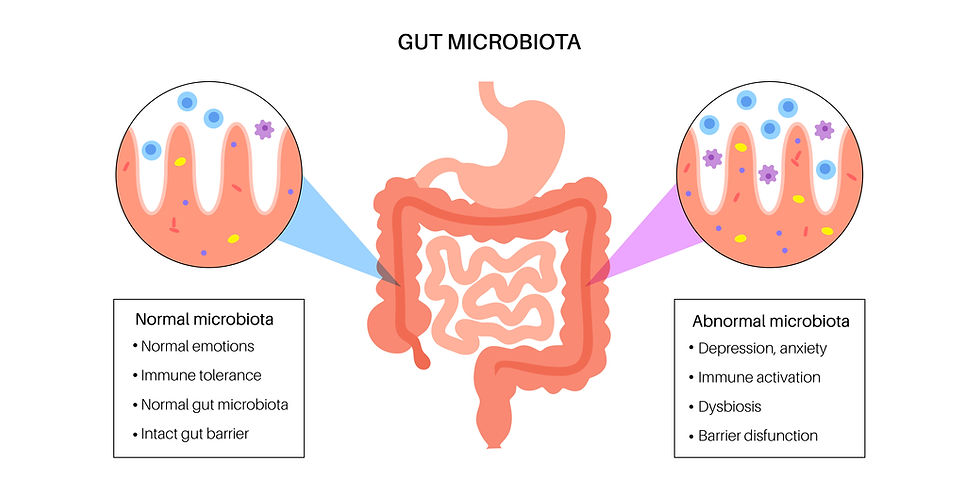
This bacterial proliferation, coupled with the potential for bacterial translocation across a compromised intestinal wall, can then worsen the initial systemic illness, potentially leading to a self-perpetuating cycle of systemic inflammation and GI hypomotility. This highlights that paralytic ileus is often not an isolated digestive issue but a powerful clinical indicator of a systemic crisis (Rao & Gershon, 2016).

A similar progression can be observed when a mechanical obstruction precedes a paralytic state. An initial physical blockage leads to bowel distension and stasis proximal to the obstruction. The stagnant ingesta becomes a breeding ground for bacteria, and the continuous pressure from the distension can lead to a compromised intestinal wall. This compromised barrier facilitates bacterial translocation and the production of an inflammatory exudate, leading to peritonitis. This severe inflammatory response then triggers the functional motor paralysis that defines paralytic ileus, transforming a straightforward mechanical problem into a complex, multifaceted condition requiring both surgical and medical intervention (Keller et al., 2018).
The interplay between systemic illness and GI stasis, therefore, forms a vicious cycle that can worsen both conditions .
How Common is it in Companion Animals?
The true incidence of paralytic ileus in companion animals is difficult to quantify because it is usually a secondary or transient disorder rather than a primary disease. Unlike mechanical obstruction, which has clearer diagnostic criteria and more readily available epidemiological data, paralytic ileus often resolves once the underlying cause is addressed, making it less frequently reported in prevalence studies (Hall & German, 2022).
While exact statistics are not available in veterinary medicine, parallels can be drawn from human medicine, where post-operative ileus occurs in up to 10–30% of patients undergoing major abdominal surgery (Vather et al., 2013). In small animals, this complication is considered frequently encountered in clinical practice, especially in referral centers where complex gastrointestinal and abdominal procedures are performed (Nelson & Couto, 2020).
Which Breeds are Predisposed?
While ileus is not typically associated with specific breeds, there is evidence that certain dog breeds, especially larger ones, may have a higher risk of postoperative complications, including ileus. Certain predispositions increase the likelihood in specific breeds:
Large and giant breeds (Great Danes, German Shepherds, Irish Setters) are at higher risk post-operatively due to greater likelihood of undergoing abdominal surgery for conditions such as gastric dilatation-volvulus (GDV) (Hall & German, 2022).
Brachycephalic breeds (Bulldogs, Pugs) are prone to post-anesthetic complications, including ileus, because of their unique anesthetic risks and frequent surgical interventions.
German Shepherds and Doberman Pinschers may have a higher predisposition to pancreatitis and peritonitis, which are key predisposing factors (Washabau & Day, 2013).
Toy breeds (Yorkshire Terriers, Pomeranians) are particularly sensitive to electrolyte disturbances and hypoglycemia, which can precipitate ileus.
In cats, Persian cats have been documented to experience chronic intestinal pseudo-obstruction, a related motility disorder, but general paralytic ileus has no strong breed link.

Causes of Paralytic Ileus in Dogs
Several pathophysiological mechanisms can contribute to the development of paralytic ileus:
Post-operative ileus

A frequent complication after abdominal surgery. This is one of the most common causes, where the manipulation of the intestines, anaesthesia, and opioid analgesia all trigger a transient disruption of the gut's normal motility, contributing to reduced peristaltic activity. (Firth, 2020).
Inflammation and infection

Conditions such as peritonitis, parvoviral enteritis, and pancreatitis induce local or systemic inflammation triggering the release of inflammatory mediators (e.g., cytokines, prostaglandins) that impair intestinal smooth muscle function (Willard & Twedt, 2019).
Electrolyte imbalances

Electrolyte imbalances, particularly low potassium (hypokalemia), low calcium (hypocalcemia) or metabolic acidosis can interfere with the nerve impulses and muscle contractions that control peristalsis reducing smooth muscle excitability and contractility (Washabau & Day, 2013).
Drug effects

Opioid pain relievers (morphine, fentanyl), anticholinergics, and certain anesthetics are well documented to have inhibitory effects on intestinal motility (Boothe, 2018).
Systemic disease or Severe infections and sepsis

Body-wide infections can disrupt motility. Sepsis, hypovolemic shock, or systemic inflammatory response syndrome (SIRS) commonly suppress vagal stimulation of the gut (Nelson & Couto, 2020). Conditions such as kidney failure, neurological disorders, or severe trauma can also lead to ileus.
Stress and pain

Severe sympathetic stimulation during pain, trauma, or stress can inhibit parasympathetic signals essential for peristalsis.
Foreign bodies
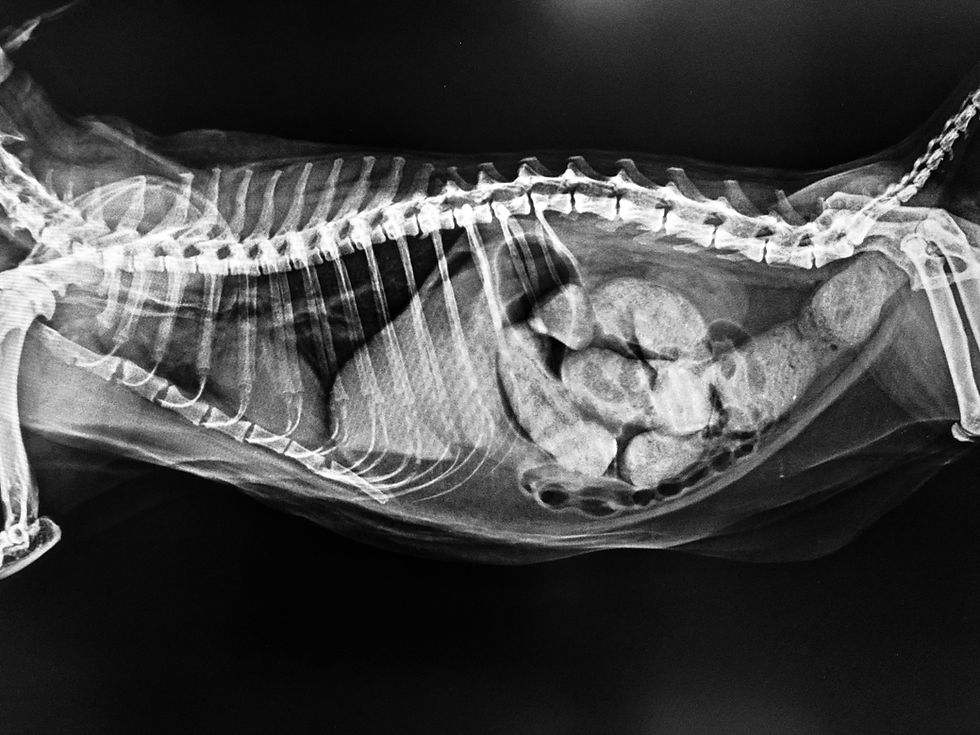
A mechanical obstruction that persists can eventually lead to a secondary paralytic ileus.
Other conditions
Other potential causes, such as severe shock, heavy metal toxicity, or abdominal injury need to be ruled out by a veterinary surgeon.
Clinical Signs
The clinical signs of paralytic ileus are non-specific and can overlap with a wide range of other GI disorders, making a definitive diagnosis based on history and physical exam alone challenging. Common signs include:
Loss of appetite (anorexia),
Vomiting or regurgitation,
Depression,
Lethargy.
On physical examination, mild to moderate abdominal distension (bloat) and discomfort upon palpation are frequently noted.
A key differentiating finding is the absence or significant reduction of normal gut sounds upon abdominal auscultation, reflecting the functional paralysis of the intestine, a key differentiator from mechanical ileus (Vather & Bissett, 2013).
In some cases, paradoxical diarrhea may be observed, particularly in partial obstructions
Chronic cases may be associated with emaciation and fever.
Absence of defecation or very scant feces
Dehydration and electrolyte imbalance signs (sunken eyes, poor skin turgor)
Diagnosis
A definitive diagnosis of paralytic ileus requires a systematic diagnostic approach to rule out a mechanical obstruction and identify the underlying cause (Hall & German, 2022).
Step 1. History Taking

A comprehensive evaluation of a patient begins with a thorough history and clinical examination. These steps are critical because ileus presents with non-specific signs that overlap with mechanical obstruction (Nelson & Couto, 2020).
Key factors to consider include:
Recent Surgical Interventions

Surgical instruments Information about any surgeries, including type, date, and complications, is essential for assessing healing and identifying potential postoperative issues. Abdominal operations (e.g., GDV correction, intestinal resection) often predispose to post-operative ileus. Post-operative ileus is one of the most common complications following abdominal procedures in small animals (Firth, 2020).
Medication History

Assorted medical drugs Details regarding current medications, dosages, and adherence can reveal drug interactions or side effects that may contribute to the condition. Drugs such as opioids, anticholinergics, and anesthetics are well recognized to suppress gastrointestinal motility (Boothe, 2018).
Systemic Illnesses

A Sick dog Chronic conditions such as diabetes mellitus, renal insufficiency, or autoimmune disorders can complicate management. Such systemic illnesses may predispose patients to electrolyte imbalances or inflammatory responses that inhibit peristalsis and delayed gut function (Washabau & Day, 2013).
Onset and Progression of Signs
Timeline of vomiting, appetite loss, or bloating helps distinguish functional vs. mechanical obstruction.
In summary, a meticulous approach to gathering a patient’s history and conducting a clinical examination is vital for accurate diagnosis and effective management of paralytic ileus in companion animals. This ensures that care is tailored to the individual needs of each patient and reduces the risk of misdiagnosis with mechanical obstruction (Willard & Twedt, 2019).
Step 2. Physical and laboratory examination

A thorough physical examination is the first step, with careful abdominal palpation and auscultation to assess for pain, distension, and the character of gut sounds. Routine laboratory work, including a complete blood count (CBC), serum biochemistry profile, and urinalysis, is essential for identifying systemic disease. These tests can reveal critical information about the underlying cause, such as electrolyte derangements (e.g., hypokalaemia, hypochloraemia, hyponatraemia), which are a primary finding in paralytic ileus cases. (Kadam et al., 2019). Serum amylase, lipase, and trypsin-like immunoreactivity measurements may help to rule out pancreatitis. In contrast, screening for electrolytes, particularly potassium and urea, should be performed to rule out a metabolic cause of ileus (Harkin & Simpson, 2025).
Step 3. Diagnostic Imaging
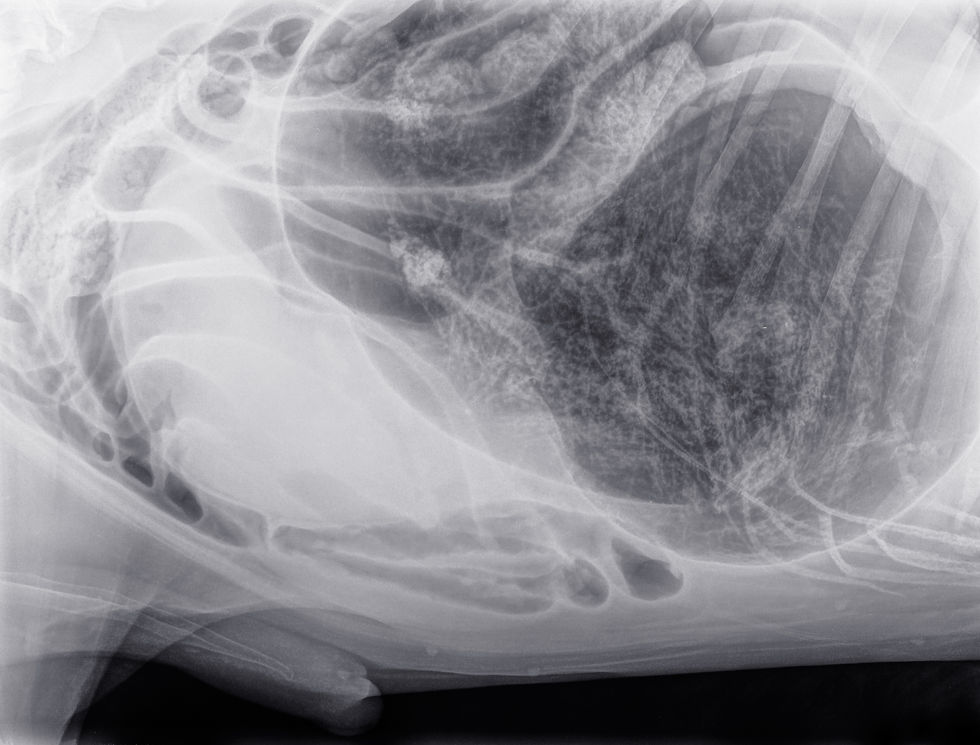
Diagnostic imaging is the cornerstone of differentiating between functional and mechanical ileus. Radiography and ultrasonography provide complementary information. Radiographs of a patient with paralytic ileus typically show a generalized and uniform gaseous or fluid distension of both the small and large intestines. This diffuse pattern is a critical distinction from the focal or segmental dilation seen proximal to a mechanical obstruction (Penninck & d’Anjou, 2015). Other radiographic findings may include a generalized decrease in serosal detail, consistent with abdominal effusion or peritonitis.
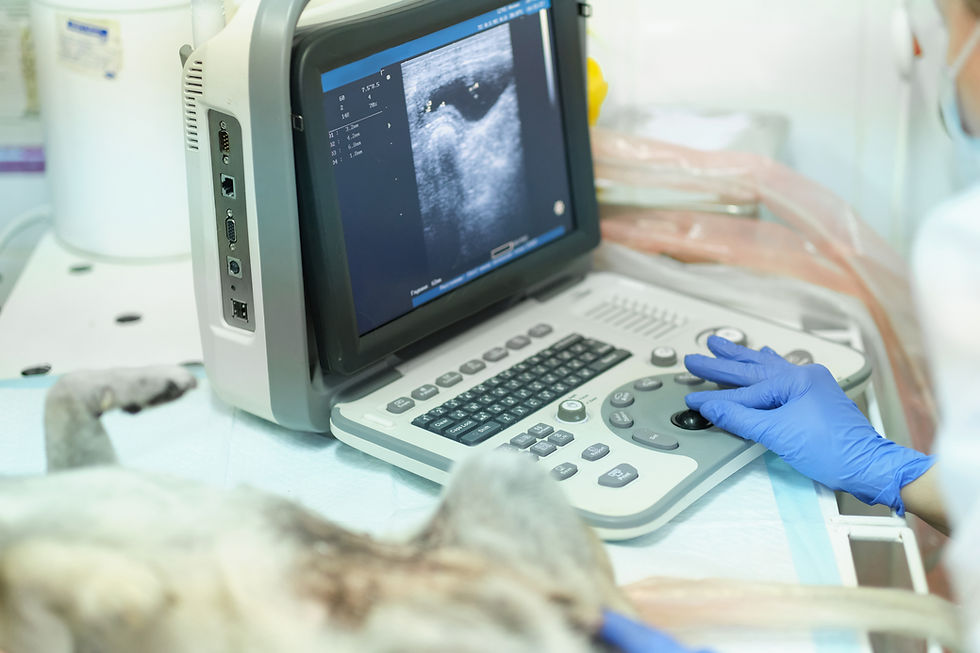
Abdominal ultrasonography provides dynamic, real-time information about intestinal motility. The key sonographic criteria for paralytic ileus are the observation of fluid-filled intestinal loops with absent or significantly decreased peristalsis (Firth, 2020). This stands in stark contrast to the non-uniform peristalsis and "pendulous movement" of ingesta often seen with a mechanical obstruction (Gaschen, 2011). The comparison of these key imaging findings is fundamental to clinical decision-making.
Step 4. Advanced and Confirmatory Diagnostics

When standard imaging is inconclusive, more specific tests may be necessary. Barium-Impregnated Polyethylene Spheres (BIPS) are useful oral markers that can be used radiographically to assess the extent of intestinal motility disorder and help localize the site of stasis. Endoscopy can also be used, particularly to assess for mechanical obstruction, but it is limited to the stomach and proximal duodenum. Ultimately, an exploratory laparotomy may be required as the gold standard and a final diagnostic step to definitively rule out a mechanical cause and confirm the diagnosis (Monk et al., 2009).
Table 1: Differential Diagnosis of Paralytic vs. Mechanical Ileus
Category | Paralytic Ileus | Mechanical Ileus |
Pathophysiology | Functional paralysis of intestinal motility | Physical blockage (e.g., foreign body, tumor) |
Physical Exam | The abdomen is typically aperistaltic with reduced or absent gut sounds | Abdomen may have colicky pain; borborygmi may be hyperactive initially, then absent |
Radiography | Generalized, uniform gaseous distension of the large and small intestines | Focal or segmental dilation of intestinal loops proximal to the obstruction site |
Ultrasonography | Fluid-filled loops with absent or decreased peristaltic activity | Non-uniform peristalsis and pendulous movement of ingesta |
Treatment and management
Paralytic ileus is both treated and managed with an approach dependent on the severity of the underlying cause. If left untreated, the accumulation of fluid and gas can become life-threatening. To effectively manage paralytic ileus in dogs, a multi-faceted approach addressing the underlying cause and providing supportive care is essential. Treatment and management strategies include:
Core Principles
The therapeutic strategy for paralytic ileus is guided by two core principles:
First, to identify and address the underlying cause, and
Second, to provide aggressive, multimodal supportive care to stabilize the patient and restore intestinal function. This approach recognizes that the ileus is a symptom, and successful management hinges on resolving and correcting the underlying cause and stabilizing the patient with supportive care (Vather & Bissett, 2013).
Foundational Supportive Care
Aggressive fluid and electrolyte therapy is a non-negotiable component of management. Intravenous fluid administration with a balanced electrolyte solution (e.g., Ringer's lactate) is crucial for correcting dehydration and addressing critical electrolyte derangements such as hypokalemia, which is a common and contributing factor to the condition (Kadam et al., 2019). While traditional management often involves "bowel rest," recent evidence suggests that an early return to feeding can help induce physiological motility and support the intestinal barrier function (Wehner et al., 2012). In severe cases with significant gastric distension, a nasogastric tube may be used for gastric decompression.
Pharmacological Interventions
Pharmacological interventions are aimed at stimulating intestinal motility and managing associated symptoms.
3.1 Prokinetic Agents
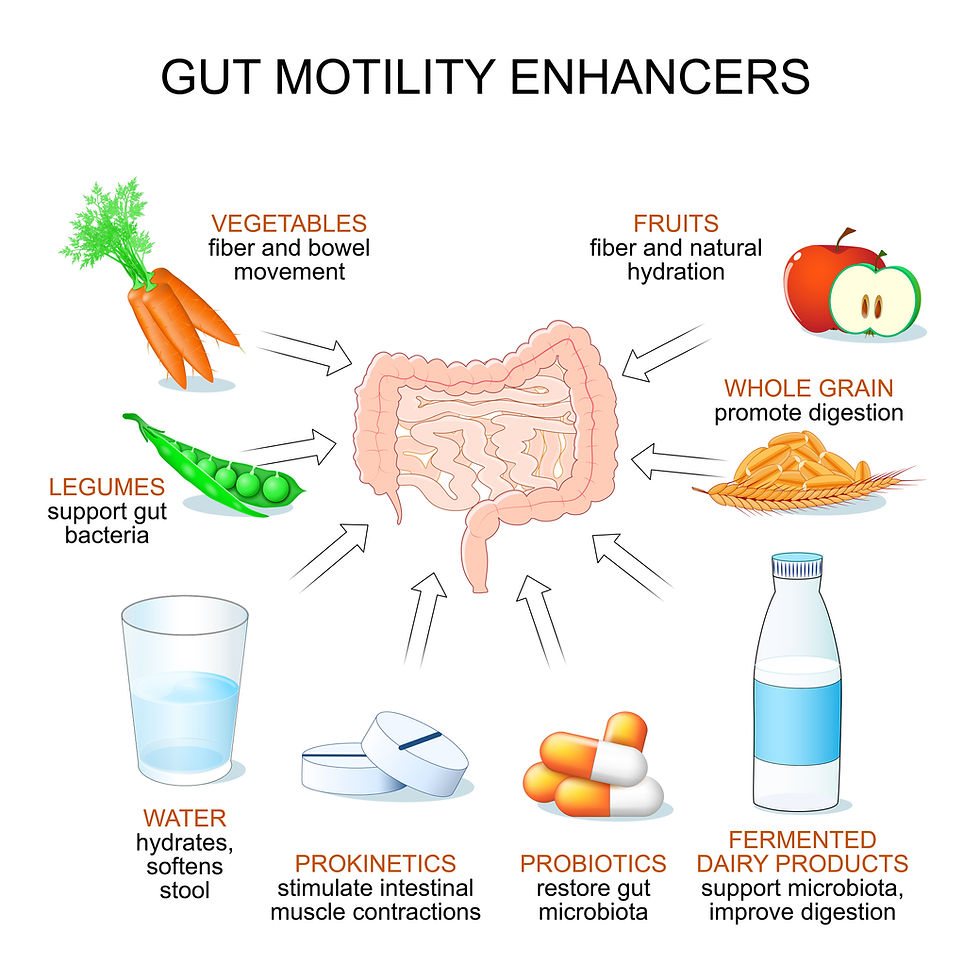
Prokinetic drugs or agents are central to therapy and the cornerstone of this approach. designed to increase the movement of ingested material through the GI tract by inducing coordinated motility patterns.
Metoclopramide: This drug is a central dopaminergic receptor antagonist and a peripheral 5-HT4 receptor agonist. It stimulates acetylcholine release in the upper GI tract, thereby increasing and coordinating motor activity in the esophagus, stomach, pylorus, and duodenum. It is a common choice for postoperative ileus and as an antiemetic, but because it crosses the blood-brain barrier, it may cause CNS side effects, antagonising central dopaminergic receptors, leading to extrapyramidal signs, including involuntary muscle spasms and motor restlessness (Flórez et al., 2018).
Cisapride: This drug is a specific 5-HT4 receptor agonist that does not cross the blood-brain barrier. Its mechanism of action involves enhancing the release of acetylcholine from the myenteric plexus, leading to increased GI motility in the esophagus, stomach, small intestine, and colon. This makes it a valuable alternative for patients susceptible to neurological side effects from metoclopramide. Cisapride is particularly useful for managing gastric stasis, idiopathic constipation, and postoperative ileus in both dogs and cats (Simmons, 2014).
Other Prokinetics: Other drugs such as erythromycin and lidocaine have also been used as prokinetics. Erythromycin, a macrolide antibiotic, can increase gastric emptying in healthy dogs by stimulating motilin receptors (Mikkelsen et al., 2014). Lidocaine is used in horses to treat postoperative ileus by its anti-inflammatory properties and prokinetic effects (Malik et al., 2021)
3.2. Other Medications
Other medications are used to address concurrent issues. Antibiotics (e.g., Sulpha-trimethoprim, Metronidazole) may be administered to address bacterial infections or prevent septic complications, particularly in cases where intestinal perforation or bacterial translocation is suspected. Antiemetics like ondansetron may also be used to control intractable vomiting by acting as a specific 5-HT3 receptor antagonist.
Table 2: Overview of Prokinetic Agents for Canine and Feline Patients
Drug | Mechanism of Action | Dosage (Dog/Cat) | Primary Indications | Key Considerations |
Metoclopramide | Dopamine receptor antagonist; 5-HT4 receptor agonist | 0.2-0.5 mg/kg, PO or SC, q8h | Postoperative ileus, vomiting, gastroesophageal reflux | Can cause extrapyramidal signs; contraindicated with GI obstruction |
Cisapride | 5-HT4 receptor agonist | Dogs: 0.1-0.5 mg/kg, PO, q8-12h; Cats: 2.5-5 mg/cat, PO, q8h | Gastric stasis, idiopathic constipation, postoperative ileus | Does not cross the blood-brain barrier; useful for chronic constipation in cats |
Erythromycin | Motilin receptor agonist | Dogs: 0.5-1 mg/kg, PO, q8-12h | Gastric emptying rate in healthy dogs | Large food chunks may enter the small intestine, inadequately digested |
Ranitidine | H2-receptor antagonist, weak prokinetic effect | 1-2 mg/kg, PO, q12h | Gastroesophageal reflux, gastric stasis | Often used in combination with other prokinetics |
Nizatidine | H2-receptor antagonist, weak prokinetic effect | 2.5-5 mg/kg, PO, q12h | Gastric stasis | Similar action to Ranitidine |
Lidocaine | Neurogenic and anti-inflammatory properties | Horses: 1.3 mg/kg bolus, then 0.05 mg/kg/min CRI | Postoperative ileus (primary use in horses) | Optimal management is controversial |
Surgical Intervention
A central tenet of clinical management is the distinction between medical and surgical conditions. A purely functional ileus is a medical problem, but due to the life-threatening nature of a mechanical obstruction, exploratory surgery is often performed even when a functional ileus is suspected (Monk et al., 2009). The difficulty in differentiating the two using non-invasive methods necessitates this crucial decision point. By performing a laparotomy, clinicians can definitively rule out a physical blockage, which would require immediate surgical intervention. In rare cases of functional ileus, surgery may be used as a therapeutic measure to decompress the severely dilated bowel, thereby relieving reflex inhibition of motility caused by the extreme distension. This highlights the critical juncture in veterinary practice where diagnostic uncertainty outweighs the theoretical non-surgical management of the condition.
Nutritional support
Bowel rest
Withholding food and water temporarily allows the digestive tract to rest. Oral feeding is reintroduced cautiously once vomiting has stopped and gastrointestinal motility has improved, because early enteral nutrition, where the gut is utilized as soon as safely possible, is beneficial
Enteral feeding
For patients who are still anorexic (have lost appetite) or hyporexic (have reduced appetite), a feeding tube (e.g., nasoesophageal, esophagostomy) may be placed to provide a highly digestible, low-fat diet. Enteral feeding helps maintain the gut barrier and stimulates motility.
Parenteral nutrition
In cases where enteral (intestinal) feeding is not an option, total or partial parenteral nutrition may be used to provide nutrients intravenously.
Transitioning back to the oral diet
Once the ileus resolves, a bland, low-fat diet can be introduced in small, frequent meals before gradually returning to the dog's normal diet.
Patient monitoring and follow-up
Frequent monitoring
Hospitalized dogs require close monitoring of hydration status, electrolytes, blood pressure, and overall demeanor.
Auscultation of gut sounds
While controversial as a sole diagnostic tool, monitoring the return of normal gut sounds can indicate improved motility.
Physical examination
The abdomen should be palpated regularly to assess for distension, pain, and the resolution of the ileus.
Follow-up care
After discharge, follow-up veterinary visits are necessary to ensure a full recovery. Long-term management may be needed for chronic underlying conditions.
Home-Based Management
Once the dog is discharged, ongoing management is essential to prevent relapse.
Dietary Management
Small, frequent meals of easily digestible, low-fat, highly digestible diets (boiled chicken/rice, prescription GI diets).
Gradual return to normal feeding, avoiding abrupt dietary changes.
Adequate hydration: fresh water available at all times.
Medication Compliance
Strict adherence to prescribed prokinetics, antibiotics (if infection present), and supplements.
Potassium or electrolyte supplementation is indicated.
Activity and Environment
Encourage gentle, controlled exercise (short walks) to promote gut motility.
Minimize stress by keeping the dog in a quiet environment.
Prevent access to foreign bodies or inappropriate foods.
Monitoring at Home
Observe for vomiting, bloating, anorexia, or lack of stool.
Monitor water intake and urination (hydration indicators).
Check for abdominal discomfort (guarding, whining).
Follow-Up Care
Return for scheduled veterinary rechecks (abdominal palpation, blood tests, or imaging).
Contact the vet immediately if symptoms return or worsen.
Prognosis
The prognosis for paralytic ileus in dogs and cats is variable and depends largely on the underlying cause, severity, and timeliness of intervention. When the root cause is reversible—such as postoperative ileus or electrolyte imbalances—most animals respond well to prompt medical therapy, and the prognosis is generally favorable (Hall & German, 2022). In contrast, cases associated with severe systemic disease such as sepsis, peritonitis, or complicated pancreatitis, are considered more serious, with recovery sometimes requiring up to two weeks of intensive treatment and carrying a guarded prognosis (Willard & Twedt, 2019).
If paralytic ileus arises from irreversible or progressive conditions, including degenerative neural disorders or advanced neoplasia, the long-term outlook is poor and may necessitate euthanasia to preserve quality of life (Nelson & Couto, 2020). Regardless of cause, close monitoring and supportive care are essential, as untreated ileus can lead to life-threatening complications (Washabau & Day, 2013).
Potential Complications of Paralytic Ileus
If paralytic ileus is not promptly treated, it can progress to life-threatening complications. One of the earliest risks is persistent vomiting and intraluminal fluid sequestration, leading to severe dehydration, electrolyte imbalances, and acid–base disturbances. These changes impair normal cellular function and may worsen systemic illness, particularly stressing the cardiovascular and renal systems (Hall & German, 2022; Washabau & Day, 2013; Nelson & Couto, 2020).
As the condition advances, marked intestinal distension may compromise mucosal blood flow, causing ischemia and necrosis of the bowel wall (Firth, 2020). Without intervention, devitalized tissue is prone to perforation, which allows intestinal bacteria and endotoxins to leak into the abdominal cavity. This often results in septic peritonitis and hypovolemic shock, both of which carry a high risk of mortality (Willard & Twedt, 2019).
Septic peritonitis demands urgent surgical correction, intensive fluid resuscitation, and broad-spectrum antimicrobial therapy. Even with aggressive treatment, the prognosis remains guarded (Hall & German, 2022).
Overall, these potential complications emphasize the need for early recognition, accurate diagnosis, and timely intervention, as prompt supportive care not only improves recovery rates but also prevents progression to catastrophic outcomes (Firth, 2020).
Species-Specific Considerations: Dogs vs. Cats

Although the core pathophysiology of paralytic ileus is similar across species, dogs and cats differ in predispositions and management needs (Hall & German, 2022).
Dogs are prone to ingesting large, indigestible foreign bodies such as bones, toys, or rocks, making mechanical obstruction a frequent precursor to ileus (Nelson & Couto, 2020). Large breeds, including German Shepherds and Great Danes, are also predisposed to GDV, which is often complicated by post-operative ileus (Washabau & Day, 2013).
Cats, in contrast, commonly ingest linear foreign bodies like string or yarn, which can cause intestinal plication and secondary ileus (Willard & Twedt, 2019). Careful oral examination is critical, as such material is often anchored at the base of the tongue (Hall & German, 2022).
Pharmacological choices also vary. Cisapride is particularly valuable in cats with chronic constipation or megacolon, sometimes delaying the need for colectomy, while metoclopramide and low-dose erythromycin are more widely used prokinetics in dogs (Boothe, 2018).
References
Boothe, D. M. (2018). Small Animal Clinical Pharmacology and Therapeutics. Elsevier Health Sciences.
Firth, A. M. (2020). Post-operative ileus in companion animals: recognition and management. Journal of Small Animal Practice, 61(3), 147–156.
Furness, J. B. (2012). The enteric nervous system and neurogastroenterology. Nature Reviews Gastroenterology & Hepatology, 9(5), 286–294. https://doi.org/10.1038/nrgastro.2012.32
aschen, L. (2011). Ultrasonography of small intestinal motility in dogs and cats. Veterinary Clinics of North America: Small Animal Practice, 41(2), 329–344. https://doi.org/10.1016/j.cvsm.2010.12.004
Grundy, D., Al-Chaer, E. D., Aziz, Q., Collins, S. M., Ke, M., Taché, Y., & Wood, J. D. (2018). Fundamentals of neurogastroenterology: Basic science. Gastroenterology, 154(2), 279–294. https://doi.org/10.1053/j.gastro.2017.07.046
Hall, E. J., & German, A. J. (2022). BSAVA Manual of Canine and Feline Gastroenterology (3rd ed.). BSAVA.
Harkin, K., & Simpson, J. (online). Ileus. In Vetlexicon Canis. Vetstream Ltd. https://www.vetlexicon.com/treat/canis
Kadam, R., Bhosale, S., & Shinde, A. (2019). Electrolyte imbalance in gastrointestinal motility disorders. Journal of Clinical and Diagnostic Research, 13(5), OE01–OE05. https://doi.org/10.7860/JCDR/2019/40812.12856
Kalff, J. C., Schwarz, N. T., Walgenbach, K. J., Schraut, W. H., & Bauer, A. J. (2013). Leukocytes of the intestinal muscularis: Their role in postoperative ileus. Gut, 52(3), 460–468. https://doi.org/10.1136/gut.52.3.460
Keller, J., Bassotti, G., Clarke, J., Dinning, P., Fox, M., Grover, M., & Tack, J. (2018). Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nature Reviews Gastroenterology & Hepatology, 15(5), 291–308. https://doi.org/10.1038/nrgastro.2018.7
Luckey, A., Livingston, E., & Tache, Y. (2003). Mechanisms and treatment of postoperative ileus. Archives of Surgery, 138(2), 206–214. https://doi.org/10.1001/archsurg.138.2.206
Malik, M., Syed, M. H., & Khan, S. A. (2021). Intravenous lidocaine in veterinary medicine: Uses and controversies. Veterinary Anaesthesia and Analgesia, 48(4), 507–515. https://doi.org/10.1016/j.vaa.2021.01.004
Miedema, B. W. (2016). Pathophysiology and management of postoperative ileus. Surgical Clinics of North America, 96(5), 1127–1141. https://doi.org/10.1016/j.suc.2016.05.002
Mikkelsen, K., Rønnestad, I., & Haugen, J. (2014). Erythromycin as a prokinetic drug in veterinary medicine. Acta Veterinaria Scandinavica, 56(1), 67–75. https://doi.org/10.1186/s13028-014-0067-2
Monk, J., Hall, E. J., & German, A. J. (2009). Small animal gastroenterology. Saunders Elsevier.
Nelson, R. W., & Couto, C. G. (2020). Small Animal Internal Medicine (6th ed.). Elsevier.
Penninck, D., & d’Anjou, M. A. (2015). Atlas of small animal ultrasonography (2nd ed.). Wiley-Blackwell.
Rao, M., & Gershon, M. D. (2016). The bowel and beyond: The enteric nervous system in neurological disorders. Nature Reviews Gastroenterology & Hepatology, 13(9), 517–528. https://doi.org/10.1038/nrgastro.2016.107
Sharkey, K. A. (2015). Emerging roles for enteric glia in gastrointestinal disorders. Nature Reviews Gastroenterology & Hepatology, 12(9), 485–496. https://doi.org/10.1038/nrgastro.2015.90
Simmons, R. (2014). Clinical applications of cisapride in small animal medicine. Journal of Veterinary Pharmacology and Therapeutics, 37(3), 209–216. https://doi.org/10.1111/jvp.12086
Vather, R., & Bissett, I. (2013). Management of prolonged post-operative ileus: Evidence-based recommendations. ANZ Journal of Surgery, 83(5), 319–324. https://doi.org/10.1111/ans.12002
Washabau, R. J., & Day, M. J. (2013). Canine and Feline Gastroenterology. Elsevier Health Sciences.
Wehner, S., Behrendt, F. F., Lyutenski, B. N., Lysson, M., Bauer, A. J., & Hirner, A. (2012). Inhibition of intestinal inflammation by experimental prokinetic therapy in mice. Gastroenterology, 142(2), 389–398. https://doi.org/10.1053/j.gastro.2011.10.046
Willard, M. D., & Twedt, D. C. (2019). Gastrointestinal, Pancreatic, and Hepatic Disorders. In: Ettinger & Feldman (Eds.), Textbook of Veterinary Internal Medicine (8th ed.). Elsevier.

























Comments